Professor in Residence, Department of Architecture, GSD, Harvard University, Cambridge MA, USA
Identifying materials that can substitute rare natural resources is one of the key challenges for improving resource efficiency in the building sector. With a growing world population and rising living standards, the amount of salt (sodium chloride) produced as waste through seawater desalination and potash mining processes is increasing. Unfortunately, most of it is disposed of into nature where it causes environmental pollution. On the other hand, salt is affordable and can be used therapeutically in various respiratory treatments and to store humidity and heat. It was, therefore, necessary to determine salt materials already in use in building construction. The aim of this research was to identify those that have been used in history and analyzed in scientific studies, to investigate their physical and mechanical properties, and to identify the most promising applications in the construction field. This was accomplished via literature review, classifying the salt materials into three groups (raw salt, composite salt, and processed salt). It was found that salt has been used as a building material for centuries and has potential for future applications.
Salts 1,2,3,4,5 are defined in chemistry as compounds of cations (positively charged ions) and anions (negatively charged ions). Sodium chloride (NaCl), or common salt, is the most known of all salts and consists of Na (Sodium) and Cl (Chloride) ions. It can be found in natural underground deposits as salt rocks (halite) or in seawater or brine as dissolved constituents. Salt rocks were formed through many geologic periods by tectonic movement and pressure on salt evaporation ponds (crystallization of salt in saline water by solar evaporation).6,7 Dissolved salt particles are gained back from seawater or brine through natural solar evaporation (salt ponds) and mechanical evaporation.8,9
However, other sources of salt are emerging as a result of the growing world population:10 salt from desalination plants and from potash mining. Fresh water is produced from seawater in desalination plants, with a discharge into the sea of around 142 million m3 [5,014,682,674 ft.3] of salt brine waste per day.11 A similar situation 12 is observed in intensive agriculture, where nutrients are added to the soil to speed up plant growth. One of these fertilizers is potash and for its production a high amount of salt waste is created (between 51,742.98 m3 [1,827,286 ft.3]/day and 436,956.29 m3 [15,430,965 ft.3]/day).13 The negative environmental impacts of salt waste discharge such as change of salinity, increase in temperature, and loss of biodiversity in both maritime and freshwater environments, have been acknowledged in many scientific studies.14,15,16 With this in mind, using salt as a building material may increase resource efficiency while reducing the salt contamination of flora and fauna.
On the other hand, salt is an affordable natural product 17,18 and can store humidity 19,20,21 and heat.22,23,24,25,26 In terms of material humidity, Feldman 27 described NaCl as a hygroscopic material with a critical point of solubility of 75.3% relative room humidity at 20 ⁰C room temperature. The specific heat capacity of sodium chloride in its pure state varies from 0.853 J/(gK) 28 to 0.859 J/(gK).29,30 Salt can deactivate the growth of microorganisms 31 and is used not only therapeutically in various respiratory treatments 32,33,34,35,36 but also for skin diseases such as atopic dermatitis 37 or human lung cancer.38
For 7,000 years salt was mostly used in cooking and the conservation of food and later for trading, while currently it can be found in 14,000 different applications in the chemical and food industry.39 However, salt in the field of building construction is associated with damage in masonry. With the increasing water content in brick masonry, salt dissolves and moves together with water through the capillary pores. In drier and hotter climate conditions, the water evaporates and salt crystalizes. Salt crystallization in the capillary pores produces an interior pressure and can cause deterioration and efflorescence of the brick.40
Due to its salt-related problems in masonry, its high value in history, legally restricted availability, high porosity, and dissolution in water, salt is still not fully accepted as a building material. However, examples exist of salt building materials used in various applications: protecting the disposal of radioactive waste,41,42 defending Roman harbors,43,44,45 for building houses in deserts,46,47,48,49,50 and optimizing indoor air quality.51,52,53,54,55,56,57,58,59,60,61
Salt building materials have further potential advantages in increasing resource efficiency; they have attractive hygrothermal properties, are not inflammable, and are antibacterial. However, no general analysis of salt’s application as a building material and its properties exists. Thus our study investigates the potential and limitations of using salt in building construction.
The state of the art is organized in a two-group framework that shows salt at two different levels. At the micro level, the basic information about salt (classification, crystallization, and physical and chemical material properties) is provided without specific application purposes. At the meso level, the research is about raw salt, composite salt, and processed salt, shown at the level of a loose product. This review study revealed that there was almost no research on the topic of salt building materials and thus opens up research areas that merit investigation.
PROPERTIES OF SALT (NaCl)
“Sodium is the sixth most abundant element in the Earth’s crust” 62 and its compound sodium chloride the most known in human society. Most properties of salt depend on the production process and geological history and the purity and content of other minerals, as well as interactions between the particles and pore spaces. Table salt or sodium chloride, NaCl, is a compound of two ions: Na (sodium) and Cl (chloride). A bond between these two ions is called an ionic bond, where electrons from one atom are transferred to another.63,64 All ions are connected in a cubic lattice, which can have any number of ions depending on the size of the crystal.65 Salt crystals (Fig. 1) are transparent and colorless, and the crystalline powder is white.
Different studies show that the permeability and porosity of salt are low 66 due to a lack of open spaces within the material. Salt is applied for sensitive heat storage in solar power plants 67 due to its high melting point.68,69 If small impurities such as anhydrite, gypsum, dolomite, calcite, pyrite, quartz, or iron oxides are present in salt, the bulk density value (from 2.17 g/cm³ to 2.25 g/cm³ at 20 ⁰C) and specific heat capacity (from 0.853 J/(gK) to 1.224 J/(gK)) are increased.70,71,72,73 Salt is soluble in water, methanol, or formic acid, but not in ethanol or glycerol.74 In terms of material humidity, at more than 75.3% relative humidity,75 the pores in salt are filled with water, the crystals start to dissolve and brine is formed. If relative humidity decreases, the brine evaporates and re-crystallization takes place. This repeating process within salt generates strong connections between crystals and throughout history, therefore, has been used to create strong materials. The detailed chemical and physical properties of salt are listed in Table 1.
TYPES OF SALT MATERIALS
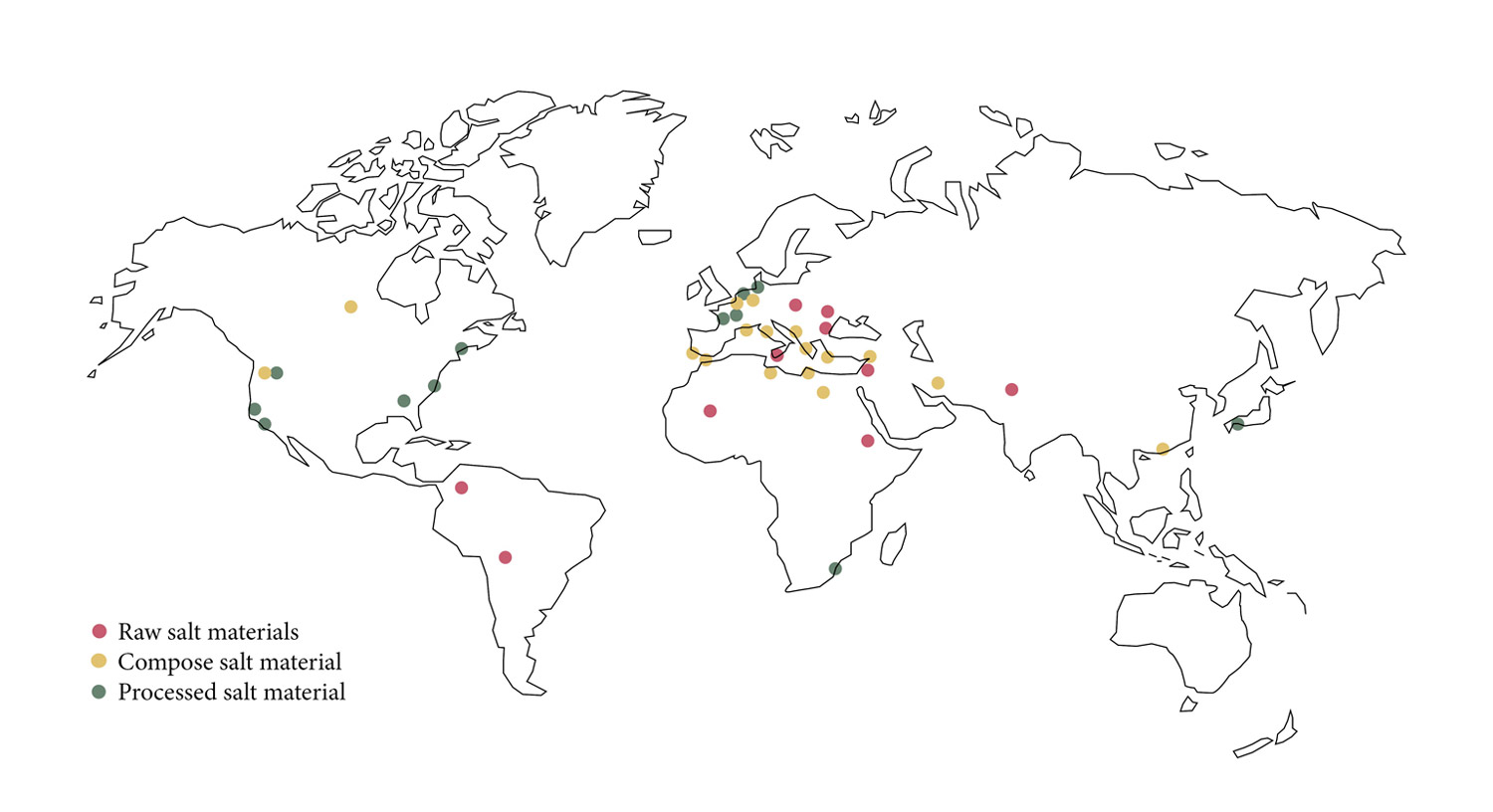
Salt materials in this paper are divided into three groups (Fig. 1): raw salt materials (RS), composite salt materials (CS), and processed salt materials (PS). Raw salt materials consist of solid salt blocks with the highest NaCl content (Table 2) extracted directly from nearby salt lakes.76,77,78 Romans mixed seawater, volcanic ash, and lime to create extremely durable concrete despite constant exposure and contact with seawater.79 This and other materials mixed with a salt content of up to 95% (Table 2) is classified as CS material. Processed salt materials are materials in which salt is compressed 80,81,82 or which use natural evaporation of salt solution for growing NaCl crystals.83,84,85,86,87
Raw Salt Materials
Knowledge about RS materials is based on very limited historical data. Most historical examples have in common severe climate (very hot and dry), specific location (near salty lakes and salt mines), and unavailability of other local construction materials. Pliny the Elder wrote in the 1st century A.D. about the city called Getta, apparently located in the region of Pella and Damascus as being “fortified with towers made of square masses of Salt.” 88 In the 14th century the Moroccan traveler Ibn Battuta describes the city Taghaza in Mali as “a village with no attractions. A strange thing about it is that its houses and mosques are built of blocks of salt and roofed with camel skins. There are no trees, only sand in which there is a salt mine.” 89
Another abandoned town in the Danakil Depression in Ethiopia is Dallol which shows destroyed walls built in the past from salt blocks.90 The single-story walls of the buildings were built of irregular medium-format salt blocks cut from the surrounding area and bonded with a mortar. Salar de Uyuni in Bolivia is the largest salt flat on Earth located above sea level and its salt crust consists of 95% halite and 5% fine-grained gypsum.91 This monolith-like salt stone is cut from the ground, shaped, and used as a construction material for buildings. One of these buildings, which is built out of some one million salt blocks, is the hotel Palacio de Sal.92 Other RS materials are rock salts from salt mines 93,94,95,96,97,98,99 with bulk density from 1,920 kg/m3 to 2,160 kg/m3, low porosity (0.6–7.2%), specific heat capacity around 865 J/kgK, thermal conductivity from 2.65 to 6.65 W/(mK), widely varied hygroscopic properties due to different additive contents, and compressive strength ranging from 3.3 to 20.7 MPa (Table 3). Pink salt blocks are the most conventional rock salts 100 and are used in the interiors of spas, restaurants, and residences.
Composite Salt Materials
Both in history and today, CS materials in history have combined different local materials according to resource availability and to improve material properties. The first combinations occurred between salt and local materials such as clay or volcanic ash. Karshee or Karshif is a salt block (NaCl) enriched with clay and sand, which is first dried under direct sun exposure and then used as a “brick” in the Kershee masonry building technique found in Siwa Oasis in Egypt.101,102 Dried salt bricks from nearby salt lakes are bound with soil and salt-rich mud mortar called tilaght. In comparison to salt rocks, a Karshif stone has more impurities and shows lower values in terms of density, porosity, hygrothermal properties, and compressive strength (Table 3).
Under specific Siwa Oasis climate conditions, the movement of water between the salt blocks and the mud causes the formation of new salt crystals resulting in “a high mechanical resistance of the masonry.” 103 Analysis of these “stones” shows that the oldest blocks are composed of 95% salt.104 Compared with sandstone and limestone, Karshif stone shows the highest thermal conductivity with 1.62 W/mK and the lowest specific heat capacity with 0.71 kJ/kgK.105 However, if the environmental relative humidity exceeds 76%, the sodium chloride in the Karshif stones starts to dissolve. Conversely, at a relative humidity of less than 76% the sodium chloride crystals start to grow.106,107,108 It has been observed that under these repeated cycles of drying and wetting salt blocks and mortar transform to “a sort of monolith.” 109
Concrete is a composite material that has been used in many ancient structures. The Romans discovered how to make it very durable and waterproof for use in seawater.110 They mixed volcanic ash, lime, and seawater 111 and built robust protection walls for their harbors in the Mediterranean Sea. The chemical and mechanical properties of Roman concrete were determined with core segments drilled from ancient structures.112,113,114 More information about the influence of seawater or salt on modern concrete can be found in several studies in the literature.
In comparison to concrete mixed with fresh water, that mixed with seawater (Table 3) shows an increase in density,115,116 setting times,117,118,119 and compressive strength after seven days.120,121,122,123,124 On the other hand, the porosity 125,126 and water absorption decreased.127,128 After twenty-eight days, the long-term compressive strength 129,130,131 between both concrete mixtures is almost the same. Concrete mixed with a high amount of salt is called salt concrete and is used for the protection of natural radiation and to prevent leakage of radioactive waste in salt caves.132,133
Salt concrete and salt caves create strong bonds with each other,134,135,136,137,138 producing a more secure protection for radioactive waste. A salt concrete composition for the safe disposal of radioactive waste in Morsleben, Germany was made of cement, fly ash, water, and salt (53.8 mass-%).139 In comparison to commonly used concrete, test results of salt concrete showed lower Young’s modulus values, similarity in strength, higher porosity, and lower permeability. The values of specific heat capacity (C= 0.93 kJ/(kgK)) and heat conductivity (λ=1.14 W/(mK) of salt concrete are lower than those of concrete. More or less identical material properties were found by Czaikowski et al.140
In the last ten years, salt and starch mixtures have been used in the building area for research purposes in the field of additive manufacturing.141,142,143 Dr. Mark Ganter and his team started experimenting in 3D printing using a salt-starch compound.144 His recipe of salt (eight parts by weight) and maltodextrin (one part by weight) was later used by researchers from the University of California and by Geboers/TU Delft to create different 3D-printed salt objects 145 and to determine density, tensile, and compressive strength.146 A University of California team mixed salt and maltodextrin, bound it with rice wine and strengthened it with wax.147,148 Salt-starch 149 showed, in comparison to modern concrete, only one part to thirteen of the compressive strength and two-thirds of the density (Table 3).
Japanese researchers 150 also mixed salt with starch but then added wheat flour and dextrin. Their material was 3D-printed as a scaffold and used as a mold for other materials. When the conglomerate was soaked in water, the salt-starch dissolved and the complex structure remained. The Iranian company Emtiaz Architecture & Design Group 151 mixed salt and natural gum to create a stucco-like salt material for the walls, structural sculptures, and ceilings of a fast-food restaurant near Shiraz in Iran to imitate a salt cave environment. Material ratios have not been published in the accessible literature.
Processed Salt Materials
Processed salt materials are produced by processing technologies such as pressing or melting and by the natural processes of salt crystallization. Compressed salt materials were first manufactured as salt lick blocks 152 for livestock nutrition.153 However, the corresponding literature states that using salt lick blocks for building construction 154 cannot be sustained if the salt is not mixed with additives 155 or coated with epoxies.156 Schelven experimented with salt extracted from salt mining and salt desalination processes. In order to provide a temporary waterproof coating, salt blocks were dipped into paraffin at lower and higher temperatures as well as into epoxy for two hours at 150–350 ⁰C [302–662 °F] to develop a permanent waterproof coating.The resistance to water and humidity of salt blocks was examined by Mandler and his group in 2018,157 where a small amount (0.1–15 wt%) of an additive was added to sodium chloride. Depending on the material properties, compressed salt blocks did not completely dissolve when exposed to water. Moreover, the samples preserved the main core and exhibited compressive strength in the range of 40–70MPa (Table 3).
It was also found that when subjected to humidity, salt objects with additives did not start to dissolve below 74% relative humidity at 40 ⁰C [104 °F]. Furthermore, at 74% relative humidity the blocks held their shape for eight hours, some for even longer at higher relative humidity.Pressed salt interior design elements in the form of a marble-like set of tables and chairs have been created by the artist Roxane Lahidji 158 who pressed a mixture of salt, tree resin, and coal powder. An experimental study in 2013 159 went even further and observed how the bonds between sodium chloride with Na-ions or Cl-ions changed under extreme application of pressure and temperature conditions similar to those in the universe. It was noticed that new bonds similar to metallic bonds appeared.
Salt crystallization processes such as salt crystal growth by seawater evaporation or salt crystal growth in very salty water has mostly been used by artists and architects.160,161,162,163,164 The artist Sigalit Landau 165 sunk everyday objects into the salty Dead Sea. Karljin Sibbel 166 created small stand alone salt structures such as seats or vases by controlling the crystallization process. In 2009 Wen Ying Teh 167 suggested an observation building for flamingos made of aluminum frames and nylon ropes that absorb salt water from the lake, forming a translucent crust through the evaporation of water. A similar concept was also developed by the architectural office Faulders Studio 168 and the French architectural office Sitbon Architecture.169 A thin plant-based wall panel with a surface of salt crystals was designed by Jessica Owusu Boakye to provide good indoor climate conditions in spaces of relaxation, rest, and exercise.170
Salt in Building Applications
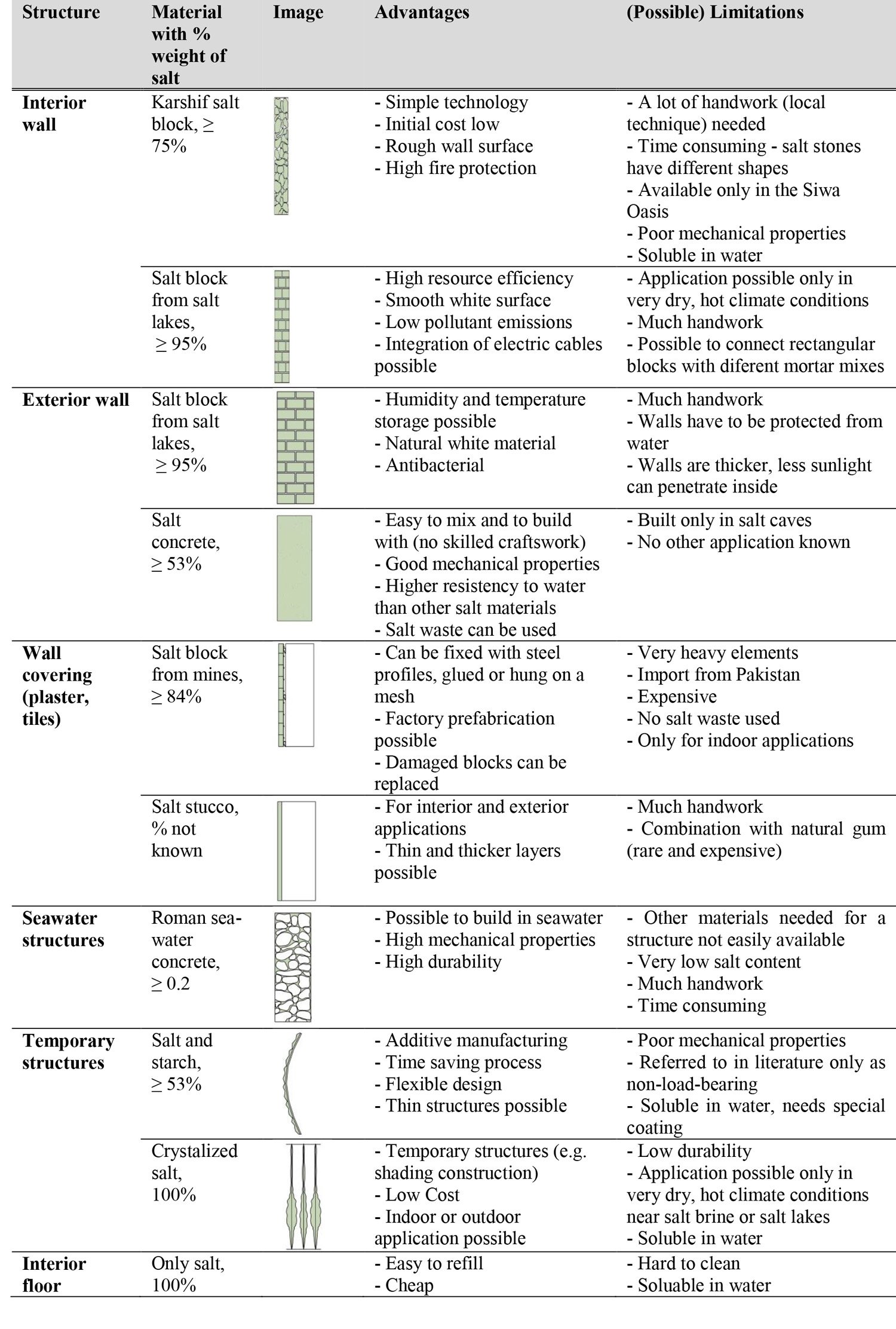
For the earlier uses of salt as a building material, climate conditions (very dry and hot), material availability, and local construction techniques were important. Initially, salt blocks were used mainly as masonry (connected with mortar) to build exterior or interior walls. However, with the development of other manufacturing techniques and material mixtures, new salt applications emerged: salt for cladding, plastering, protection of walls, and temporary constructions. In the near future, we expect salt will be used in additive manufacturing (3D printing) to save time and money. In Table 4 we consider known applications of salt materials with their target material properties and evaluate their limitations and advantages.
Many past applications are no longer applicable in contemporary practice as there may be a lack of craft knowledge, they are too time consuming, or other necessary materials are no longer available. The design criteria for the current/future use of salt in building construction should therefore be carefully considered. First, salt as well as any additional materials should be locally available. Second, depending on whether the salt construction is used outdoors or indoors, the air temperature should be at the desired level and the air humidity lower than 70%. Third, the material’s mechanical properties must be sufficient to obtain structural firmness. Finally, a high amount of salt is preferred.
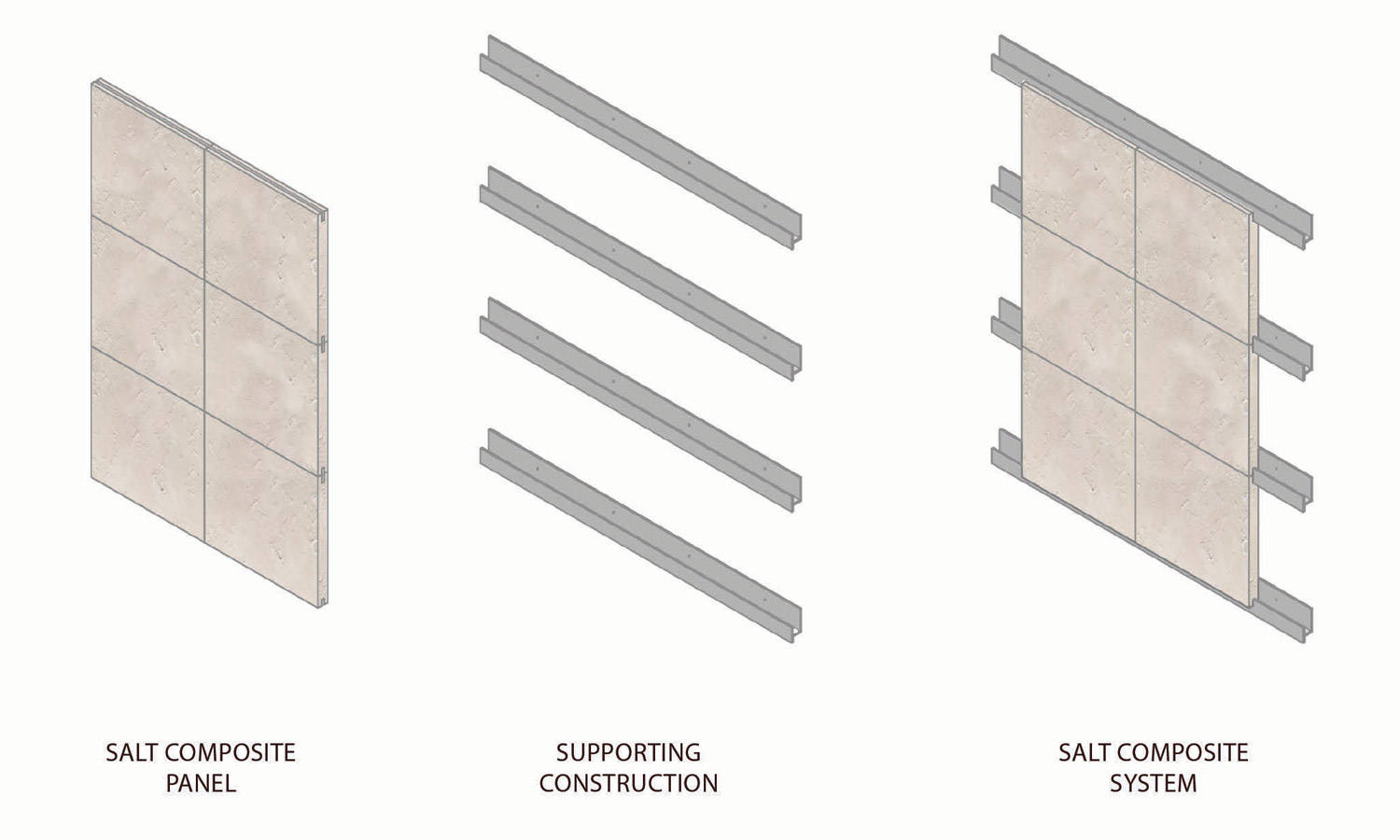
Our current research shows that the most economical way of using salt in construction is to mix it with other building materials. It can be mixed in, added to molds, or dried and fixed on a wall surface. Fig. 3 shows how to apply salt plates on a supporting construction. Another method would be to print salt binder mixes with a 3D printer to create stable exterior wall constructions and 3D elements (Fig. 4). Apart from the above-mentioned design features, these also have the advantages of durability, design freedom, economy, and construction speed.
POSSIBILITIES CHALLENGES, AND FUTURE OPPORTUNITIES
The material properties of salt such as humidity and heat storage, compressive strength, and health issues are attractive for potential applications in the building sector. Composite salt materials show a wide variety of properties when mixed with clay, concrete, or starch. Karshif salt blocks have, in comparison to other CS materials, the highest salt content; mixtures between salt and starch allow utilization in 3D printing; Roman marine concrete is known for its high durability to withstand a marine environment; and, in comparison to commonly used concrete, salt concrete showed similarity in strength and heat capacity. The manufacturing processes of pressing, surface impregnation, and natural salt crystallization show interesting results in terms of humidity exposure and architectural applications such as shading systems.
Negative effects of salt such as dissolution at high environmental relative humidity, increasing porosity, reduction of compressive strength in some material mixtures, and negative experience with salt in masonry (efflorescence and deterioration) restrain its application. The properties of existing salt materials in the building sector have only been explored to a limited degree in the literature, which has concentrated mostly on the negative impacts of salt or on the protection of salt in building materials. Therefore, knowledge about the behavior and properties of salt is often limited, as studies have investigated only one or two properties without exploring their combination. A greater overview could lead to a better understanding of salt as a building material.
This paper is the first ever review of salt materials as building materials. This indicates that salt materials need to be further analyzed and the paper suggests several recommendations for future application:
- Salt mixed with other materials can increase the resource efficiency in building construction. Currently, REA gypsum represents around 50% of the gypsum used in Germany, but this resource will soon disappear because of the planned closure of the country’s coal power plants. Therefore, mixing salt with gypsum or other materials could reduce the demand for energy-consuming or rare materials.
- Some salt materials (such as salt concrete and Karshif) can influence indoor environment conditions. Salt concrete can absorb and release heat from the air and could be used as a thermal storage material in climates with high day-night temperature differences. Karshif (salt-clay) can absorb and release the vapor from the air and could be used as a humidity storage material in climate conditions with short exposure to higher relative humidity.
- Salt materials with a higher amount of salt and increased efflorescence on the surface could positively affect human health in buildings. Emissions from salt materials could provide healthier working or living conditions. Salt is not toxic, is free of chemical emissions and has no odor.
- Raw salt materials or salt brines could be used for temporary building construction such as pavilions, exhibition construction, and shading systems of buildings and open areas. Temporary building structures in very hot and dry climate conditions could be built with the technique of salt crystal growth following seawater evaporation and could easily be demolished with water.
- Salt is not flammable and has good acoustic properties (in comparison with wood it has higher average bulk density). It could be used as infill material for walls in dry and hot climate conditions.
- Salt materials can be adapted to different building techniques (e.g., 3D printing, additive manufacturing, masonry, prefabrication).
CONCLUSION
Salt has always played a significant role in human life. Recently, salt waste generated from the desalination and potash mining industries has started to encourage its re-use in different applications. This paper has explored the historical and future use of salt in the field of construction. Resource efficiency has become one of the major concerns in the construction industry and, despite some of the limitations of salt mentioned earlier, there is still potential with further technological advancement (3D printing, additive manufacturing, pressing, melting), and material research. In general, using salt waste is a promising solution for reducing the dependence on conventional materials such as gypsum or cement, creating healthier indoor environments, and reducing pollution.
Alberto Ciferri and Angelo Perico, eds., Ionic Interactions in Natural and Synthetic Macromolecules (Hoboken N.J., USA: Wiley, 2012).
Adam Augustyn et al., eds., Ion | Definition, Chemistry, Examples, & Facts
(Encyclopædia Britannica, inc., 2019) - https://www.britannica.com/science/ion-physics, accessed May 10, 2020 .
R. M. Geertman, “Sodium Chloride: Crystallization,” in Encyclopedia of Separation Science, ed. Ian D. Wilson (San Diego CA, USA, London: Academic, 2000) -
http://www.sciencedirect.com/science/article/pii/B0122267702060610.
Jan W. Gooch, ed., Encyclopedic Dictionary of Polymers, Springer reference (New York: Springer, 2007).
Miloslav Nič et al., Compendium of Chemical Terminology (Oxford, UK: Blackwell Scientific Publications, 1997) - https://goldbook.iupac.org/.
Michael R. Hudec and Martin P. Jackson, “Terra Infirma: Understanding Salt Tectonics,” Earth-Science Reviews 82, nos. 1–2 (2007) - doi:10.1016/j.earscirev.2007.01.001.
Susan R. Feldman, “Sodium Chloride,” in Encyclopedia of Chemical Technology, eds. Raymond E. Kirk and Donald F. Othmer (New York: Wiley, 2003).
Chaoji Chen, Yudi Kuang, and Liangbing Hu, “Challenges and Opportunities for Solar Evaporation,” Joule 3, no. 3 (2019) - doi:10.1016/j.joule.2018.12.023.
Feldman, “Sodium Chloride.”
United Nations Secretariat, “Expert Group Meeting on Completing the Fertility Transition,” Population Newsletter 73 (2002) - https://www.un.org/en/development/desa/population/publications/pdf/newsl....
Edward Jones et al., “The State of Desalination and Brine Production: A Global Outlook,” The Science of the Total Environment 657 (2019) - doi:10.1016/j.scitotenv.2018.12.076.
J. E. Tallin, Dennis E. Pufahl, and Sidney Lee Barbour, “Waste Management Schemes of Potash Mines in Saskatchewan,” Canadian Journal of Civil Engineering 17, no. 4 (1990), 528–542 - doi:10.1139/l90-061
Henry Rauche, ed., Die Kaliindustrie im 21. Jahrhundert (Berlin, Heidelberg, Ger.: Springer Berlin Heidelberg, 2015).
Ahmed Musfique and Anwar Rifat, “An Assessment of the Environmental Impact of Brine Disposal in Marine Environment,” International Journal of Modern Engineering Research (IJMER) 2, no. 4 (2012) - http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.417.1405.
Pilar Palomar and Inigo. J. Losada, “Impacts of Brine Discharge on the Marine Environment: Modelling as a Predictive Tool,” Desalination, Trends and Technologies (2011) - doi:10.5772/14880.
Thomas Hoepner and Sabine Lattemann, “Chemical Impacts from Seawater Desalination Plants – A Case Study of the Northern Red Sea,” Desalination 152, nos. 1–3 (2003)- doi:10.1016/S0011-9164(02)01056-1.
Feldman, “Sodium Chloride.”
Sang B. Jeong, Ki J. Heo, and Byung U. Lee, “Antimicrobial Air Filters Using Natural Sea Salt Particles for Deactivating Airborne Bacterial Particles,” International Journal of Environmental Research and Public Health 17, no. 1 (2019) - doi:10.3390/ijerph17010190.
J. L. Aragones, E. Sanz, and C. Vega, “Solubility of NaCl in Water by Molecular Simulation Revisited,” The Journal of Chemical Physics 136, no. 24 (2012) - doi:10.1063/1.4728163.
Feldman, “Sodium Chloride.”
Nahla N. Makhlouf et al., “Hygrothermal Performance of Vernacular Stone in a Desert Climate,” Construction and Building Materials 216 (2019) - doi:10.1016/j.conbuildmat.2019.04.244.
Feldman, “Sodium Chloride.”
Aragones, “Solubility of NaCl.”
B. Håkansson and P. Andersson, “Thermal Conductivity and Heat Capacity of Solid NaCl and NaI Under Pressure,” Journal of Physics and Chemistry of Solids 47, no. 4 (1986) - doi:10.1016/0022-3697(86)90025-9.
Carles de Las Cuevas, “Pore Structure Characterization in Rock Salt,” Engineering Geology 47, nos. 1–2 (1997) - doi:10.1016/S0013-7952(96)00116-0.
Lewis H. Gevantman, Physical Properties Data for Salt, with the assistance of H. H. Li
et al. (U.S. Government printing office, 1981).
Feldman, “Sodium Chloride.”
Ibid.
Las Cuevas, “Pore Structure Characterization.”
Håkansson, “Thermal Conductivity.”
Jeong, “Antimicrobial Air Filters.”
Alina V. Chervinskaya and Nora A. Zilber, “Halotherapy for Treatment of Respiratory Diseases,” Journal of Aerosol Medicine 8, no. 3 (1995) - doi:10.1089/jam.1995.8.221.
J. Hedman et al., “The Effect of Salt Chamber Treatment on Bronchial Hyperresponsiveness in Asthmatics,” Allergy 61, no. 5 (2006) -
doi:10.1111/j.1398-9995.2006.01073.x.
Sala Horowitz, “Salt Cave Therapy: Rediscovering the Benefits of an Old Preservative,” Alternative and Complementary Therapies 16, no. 3 (2010) - doi:10.1089/act.2010.16302.
Tibor Horvath, “Speleotherapy: A Special Kind of Climatotherapy, Its Role in Respiratory Rehabilitation,” International Rehabilitation Medicine 8, no. 2 (1986) - doi:10.3109/03790798609166185.
Rachael Rashleigh, Sheree M. S. Smith, and Nicola J. Roberts, “A Review of Halotherapy for Chronic Obstructive Pulmonary Disease,” International Journal of Chronic Obstructive Pulmonary Disease 9 (2014) - doi:10.2147/COPD.S57511.
E. A. Puryshev, “The Efficacy of Speleotherapy in Atopic Dermatitis in Children,” Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury, no. 4 (1994) -
Jana Asselman et al., “Marine Biogenics in Sea Spray Aerosols Interact with the MTOR Signaling Pathway,” Scientific Reports 9, no. 1 (2019) - doi:10.1038/s41598-018-36866-3.
Feldman, “Sodium Chloride”; Geertman, “Sodium Chloride: Crystallization.”
Lisbeth M. Ottosen, Anne J. Pedersen, and Inge Rörig-Dalgaard, “Salt-Related Problems in Brick Masonry and Electrokinetic Removal of Salts,” Journal of Building Appraisal 3, no. 3 (2007) - doi:10.1057/palgrave.jba.2950074.
Oliver Czaikowski et al., “Development of Chemical-Hydraulic Models for the Prediction of Long-Term Sealing Capacity of Concrete-Based Sealing Materials in Rock Salt,” with the assistance of TIB-Technische Informationsbibliothek Universitätsbibliothek Hannover, and Technische Informationsbibliothek (TIB), GRS ([Köln u.a.]: GRS, 2018) -
DBE, “Planfeststellungsverfahren Zur Stilllegung Des Edlagers Für Radioaktive Abfälle Morsleben: Verfahrensunterlage,” 2004 - https://mule.sachsen-anhalt.de/fileadmin/Bibliothek/Politik_und_Verwaltu....
E. Gotti et al., “A Comparison of the Chemical and Engineering Characteristics of Ancient Roman Hydraulic Concrete with a Modern Reproduction of Vitruvian Hydraulic Concrete,” Archaeometry 50, no. 4 (2008) - doi:10.1111/j.1475-4754.2007.00371.x.
Marie D. Jackson et al., “Phillipsite and Al-Tobermorite Mineral Cements Produced Through Low-Temperature Water-Rock Reactions in Roman Marine Concrete,” American Mineralogist 102, no. 7 (2017) - doi:10.2138/am-2017-5993CCBY.
C. J. Brandon, M. D. Jackson, and Robert L. Hohlfelder, Building for Eternity: The History and Technology of Roman Concrete Engineering in the Sea, ed. J. P. Oleson (Oxford, UK: Oxbow Books, 2014).
L. Rovero et al., “The Salt Architecture in Siwa Oasis – Egypt (XII–XX Centuries),” Construction and Building Materials 23, no. 7 (2009) -
doi:10.1016/j.conbuildmat.2009.02.003.
Material District, “Bolivia’s Healing Salt Block Hotel” - https://materialdistrict.com/article/bolivias-healing-salt-block-hotel/, accessed March 9, 2020.
Nick Middleton, Going to Extremes: Mud, Sweat and Frozen Tears (London: Channel 4 Books, 2001).
H.A.R. Gibb and C.F. Beckingham, The Travels of Ibn Battuta: AD 1325–1354, vol. IV (London: Routledge for the Hakluyt Society, Second Series, 2017) -
Jonathan Couch, ed., Pliny’s Natural History: In Thirty-Seven Books (London: Printed for the Club by George Barclay, 1847–1848) - https://archive.org/stream/plinysnaturalhis00plinrich/plinysnaturalhis00..., accessed March 4, 2020.
Sylvia P. Beamon et al., “Speleotherapy for Asthma,” Cochrane Database of Systematic Reviews no. 2 (2001) - doi:10.1002/14651858.CD001741.
A.A. Abdullaev, K.M. Gadzhiev, and A.A. Eiubova, “The Efficacy of Speleotherapy in Salt Mines in Children with Bronchial Asthma Based on the Data from Immediate and Late Observations,” Vopr Kurortol Fizioter Lech Fiz Kult 5, 25-8 (1993) - https://www.unboundmedicine.com/medline/citation/8266663/, accessed August 13, 2020.
Chervinskaya and Zilber, “Halotherapy.”
Hedman et al., “Effect of Salt Chamber.”
Horowitz, “Salt Cave Therapy.”
Horvath, “Speleotherapy.”
Jeong, “Antimicrobial Air Filters.”
H. Lăzărescu et al., “Speleotherapy – Modern Bio-Medical Perspectives,” Journal of Medicine and Life 7, no. 2 (2014).
Puryshev, “Efficacy of Speleotherapy in Atopic Dermatitis.”
M. Skulimowski, “Behandlung der Bronchialasthmakranken in den Kammern der Steinsalzgrube in Wielczka,” [Treatment of Bronchial Asthma Patients in the Chambers of the Rock Salt Mine in Wieliczka] Archiv für physikalische Therapie 17, no. 6 (1965) - https://pubmed.ncbi.nlm.nih.gov/5885768/.
Rashleigh, “A Review of Halotherapy.”
C.P. Sánchez-Castillo and W.P.T. James, “Salt: Epidemiology,” in Encyclopedia of Human Nutrition, eds. Benjamin Caballero, Lindsay Allen, and Andrew Prentice, 3rd ed. (Amsterdam: Elsevier/Academic Press, 2013) - http://www.sciencedirect.com/science/article/pii/B978012375083900252X.
Augustyn et al., Ion.
Ciferri and Perico, Ionic Interactions.
Marc de Graef and Michael E. McHenry, Structure of Materials: An Introduction to Crystallography, Diffraction, and Symmetry, 2nd ed., (New York: Cambridge University Press, 2012) - https://books.google.si/books?id=tl5de4vA2psC.
Richard L. Beauheim and Randall M. Roberts, “Hydrology and Hydraulic Properties of a Bedded Evaporite Formation,” Journal of Hydrology 259, nos. 1–4 (2002) -
doi:10.1016/S0022-1694(01)00586-8.
National Center for Biotechnology Information, “PubChem Database: Sodium Chloride”- https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-chloride#section=Comput..., accessed March 15, 2020.
John B. Ferguson, “The Melting and Freezing Point of Sodium Chloride,” The Journal of Physical Chemistry 26, no. 7 (1922) - doi:10.1021/j150223a002, accessed November 20, 2018.
National Center for Biotechnology Information, “PubChem Database.”
Gevantman, Physical Properties Data.
Merck, “Safety Data Sheet for Sodium Chloride 102406,” Merck KGaA - https://www.merckmillipore.com/INTL/en/product/msds/MDA_CHEM-102406?Refe..., accessed June 16, 2020.
National Center for Biotechnology Information, “PubChem Database.”
Feldman, “Sodium Chloride.”
Ibid.
Ibid.
Attilio Petruccioli and Calogero Montalbano, eds., Oasi di Siwa: azioni per lo sviluppo sostenibile = Siwa Oasis Actions for a Sustainable Development (Bari: DICAR, 2011).
Couch, “Pliny’s Natural History.”
Gibb and Beckingham, The Travels of Ibn Battuta.
Jackson et al., “Phillipsite and Al-Tobermorite Mineral Cements.”
William J. van Schelven, “Construction Block of Compressed Salts Encapsulated with Epoxy Resin,” Patent US213602A, filed July 31, 1962, and issued October 12, 1965.
Daniel Mandler et al., “Compressed Salt Objects,” Patent 16/498,133, filed March 27, 2018, and issued January 30, 2020.
William B. Darcy, “Salt Block,” Patent 179,649, filed August 15, 1950, and issued June 3, 1952.
Faulders Studio, “Geotube Tower,” Faulders Studio - https://www.faulders-studio.com/GEOTUBE-TOWER, accessed June 14, 2019.
Dalia Manor et al., Sigalit Landau: Salt Years (Salzburg, Austria: Hatje Cantz Verlag GmbH & Company, 2019).
Bridgette Meinhold, “Sitbon Architects’ Dead Sea ‘Cristal’ Pavilion Is Actually Made of Salt!,” Inhabitat – Green Design, Innovation, Architecture, Green Building, December 7, 2013 - https://inhabitat.com/sitbon-architects-unveils-cristal-pavilion-made-of..., accessed June 8, 2020.
Red Dot Design Award, “Salt Crystal Wall” - https://www.red-dot.org/project/salt-crystal-wall-39105/, accessed June 8, 2020.
Karlijn Sibbel, “SEAt,” Studio Karijn Sibbel - https://karlijnsibbel.com/portfolio/seat/.
Couch, Pliny’s Natural History, accessed June 8, 2020.
Gibb and Beckingham, The Travels of Ibn Battuta.
Middleton, Going to Extremes.
François Risacher and Bertrand Fritz, “Quaternary Geochemical Evolution of the Salars of Uyuni and Coipasa, Central Altiplano, Bolivia,” Chemical Geology 90, no. 3 (1991) - doi:10.1016/0009-2541(91)90101-V.
Material District, “Healing Salt Block Hotel.”
Stephen J. Bauer, Bo Song, and Brett Sanborn, “Dynamic Compressive Strength of Rock Salts,” International Journal of Rock Mechanics and Mining Sciences 113 (2019) - doi:10.1016/j.ijrmms.2018.11.004.
W.B. Durham and A.E. Abey, Thermal Properties of Avery Island Salt to 573/sup 0/K and 50-MPa Confining Pressure (1981) - doi:10.2172/6420528.
Gevantman, Physical Properties Data.
Las Cuevas, “Pore Structure Characterization.”
Leo van Sambeek et al., eds., Salt Mechanics: Empirical and Theoretical Developments 1 (Elsevier Science Publishers B.V., 1993).
Fraunhofer IBP, “Bestimmung des Wärmedurchlasswiderstands und der Wärmeleitfähigkeit nach DIN 12664 an Salzkristallplatten: Prüfbericht” (Stuttgart, Ger., 2020).
Fraunhofer IBP, “Bestimmung Von Feuchtetechnischen Materialkennwerten” (Holzkirchen, Ger., 2020).
Qaze Muhammad Sharif, Mumtaz Hussain, and Muhammad Tahir Hussain, “Chemical Evaluation of Major Salt Deposits of Pakistan,” Chemical Society of Pakistan 29, no. 6 (2007), accessed May 5, 2020.
Abdelaziz Farouk Mohamed, “Comparative Study of Traditional and Modern Building Techniques in Siwa Oasis, Egypt: Case Study: Affordable Residential Building Using Appropriate Building Technique,” Case Studies in Construction Materials 12 (2020) - doi:10.1016/j.cscm.2019.e00311.
Petruccioli and Montalbano, Oasi di Siwa.
Ibid.
Ibid.
Makhlouf et al., “Hygrothermal Performance.”
Ibid.
Petruccioli and Montalbano, Oasi di Siwa.
Mohamed, “Traditional and Modern Building Techniques.”
Rovero et al., “Salt Architecture in Siwa Oasis.”
Brandon, Jackson and Hohlfelder, Building for Eternity.
Gotti et al., “Chemical and Engineering Characteristics.”
Brandon, Jackson and Hohlfelder, “The Brindisi Pila Reproduction,” in Building for Eternity.
Eva Vejmelková et al., “Engineering Properties of Concrete Containing Natural Zeolite as Supplementary Cementitious Material: Strength, Toughness, Durability, and Hygrothermal Performance,” Cement and Concrete Composites 55 (2015) - doi:10.1016/j.cemconcomp.2014.09.013.
Jackson et al., “Phillipsite and Al-tobermorite Mineral Cements.”
Donald F. Griffin and Robert L. Henry, The Effect of Salt in Concrete on Compressive Strength, Water Vapor Transmission, and Corrosion of Reinforcing Steel (Port Hueneme CA, USA: U.S. Naval Civil Engineering Laboratory, 1962).
Jianzhuang Xiao et al., “Use of Sea-Sand and Seawater in Concrete Construction: Current Status and Future Opportunities,” Construction and Building Materials 155 (2017) - doi:10.1016/j.conbuildmat.2017.08.130.
Ibid.
Falah M. Wegian, “Effect of Seawater for Mixing and Curing on Structural Concrete,” The IES Journal Part A: Civil & Structural Engineering 3, no. 4 (2010) - doi:10.1080/19373260.2010.521048.
Sajjad A. Mangi et al., “A Comprehensive Review on Effects of Seawater on Engineering Properties of Concrete,” Silicon (2020) - doi:10.1007/s12633-020-00724-7.
Griffin and Henry, The Effect of Salt in Concrete.
Mangi et al., “Review on Effects of Seawater.”
Tarek U. Mohammed, Hidenori Hamada, and Toru Yamaji, “Performance of Seawater-Mixed Concrete in the Tidal Environment,” Cement and Concrete Research 34, no. 4 (2004) - doi:10.1016/j.cemconres.2003.09.020.
Wegian, “Seawater for Mixing and Curing.”
Xiao et al., “Use of Sea-sand and Seawater.”
Ibid.
Erniati et al., “Porosity, Pore Size and Compressive Strength of Self Compacting Concrete Using Sea Water,” Procedia Engineering 125 (2015) - doi:10.1016/j.proeng.2015.11.045.
Griffin and Henry, The Effect of Salt in Concrete.
Xiao et al., “Use of Sea-Sand.”
Ibid.
Mohammed, Hamada, and Yamaji, “Performance of Seawater-mixed Concrete.”
Griffin and Henry, The Effect of Salt in Concrete.
William B. Heroy, “Disposal of Radioactive Waste in Salt Cavities: Report,” National Academies Press (US); (National Research Council (US) Committee on Waste Disposal. The Disposal of Radioactive Waste on Land. Washington (DC), 1957) - https://www.ncbi.nlm.nih.gov/books/NBK208696/.
Thomas J. Eyermann, Leo van Sambeek, and F.D. Hansen, “Case Studies of Sealing Methods and Materials Used in the Salt and Potash Mining Industries,” U.S. Department of Energy Office of Scientific and Technical Information technical report (United States, 1995) - doi:10.2172/188537.
DBE, “Planfeststellungsverfahren.”
Nina Müller-Hoeppe et al., “Integrität geotechnischer Barrieren: Bericht zum Arbeitspaket 9.2; vorläufige Sicherheitsanalyse für den Standort Gorleben,” 288, GRS-288 (2012).
Jonas L. Weber, “Untersuchung von Materialien zur Abdichtung des Kontaktbereichs zwischen Streckenverschlussbauwerken aus hydraulisch abbindenden Baustoffen und dem Salzgebirge” (dissertation, Fakultät für Energie- und Wirtschaftswissenschaften der, Technische Universität Clausthal, 2018) - https://dokumente.ub.tu-clausthal.de/servlets/MCRFileNodeServlet/clausthal_derivate_00000453/Db113843.pdf, accessed May 20, 2020.
Frank Schmidt-Döhl, “Dauerhaftigkeitsprognose von Salzbeton im Kontakt mit salinaren Lösungen,” in Baustoff und Konstruktion: Festschrift zum 60. Geburtstag von Harald Budelmann, eds. Reinhard Nothnagel and Heiko Twelmeier (Berlin, Heidelberg, Ger.: Springer, 2013).
Eyermann, van Sambeek and Hansen, “Sealing Methods and Materials.”
DBE, “Planfeststellungsverfahren.”
Czaikowski et al., “Development of Chemical-Hydraulic Models.”
Eric Geboers, “The Salt Project” (master’s thesis, Architecture and The Built Environment, TU Delft, 2015) - https://repository.tudelft.nl/islandora/object/uuid:528c3ddc-8a7e-4b95-8..., accessed November 20, 2019.
Mark Ganter, “Salty Parts – 3DP in Salt,” Mechanical Engineering Department at the University of Washington - http://depts.washington.edu/open3dp/2011/03/salty-parts-3dp-in-salt/, accessed May 11, 2020.
Jinhua Li et al., “3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications,” Materials Science and Engineering: R: Reports 140 (April 2020) - doi:10.1016/j.mser.2020.100543, accessed March 7, 2020.
Ganter, “Salty Parts.”
Ronald Rael and Virginia San Fratello, Printing Architecture: Innovative Recipes for 3D Printing (New York: Princeton Architectural Press, 2018).
Geboers, “The Salt Project.”
Rael and San Fratello, Printing Architecture.
Mark Kelly, “Salt, Emergence and Formation at the Dead Sea” (master’s thesis, College of Environmental Design, UC Berkeley, 2012) - https://books.google.de/books?id=xY-7AwAAQBAJ, accessed May 22, 2020.
Geboers, “The Salt Project.”
Yoji Marutani and Takayuki Kamitani, “Manufacturing Sacrificial Patterns for Casting by Salt Powder Lamination,” Rapid Prototyping Journal 10, no. 5 (2004) - doi:10.1108/13552540410562313.
“Salt Restaurant / Emtiaz Group,” Architecture Lab - https://www.architecturelab.net/salt-restaurant-emtiaz-group/, accessed March 8, 2020.
Joe Orton, “Animal Supplement Lick Blocks,” PCT/ZA2003/000129, filed September 6, 2002, and issued March 16, 2004.
Harinder P.S. Makkar, Manuel Sánchez, and Andrew W. Speedy, Feed Supplementation Blocks: Urea-Molasses Multinutrient Blocks Simple and Effective Feed Supplement Technology for Ruminant Agriculture, FAO animal production and health paper, 0254-6019 164 (Rome: Food and Agriculture Organization of the United Nations, 2007).
C. Lindner and T.F. Hammer, “Salt Feeding Device,” Patent US240271A, filed June 4, 1887, and issued May 1, 1888.
Mandler et al., “Compressed Salt Objects.”
van Schelven, “Construction Block of Compressed Salts.”
Mandler et al., “Compressed Salt Objects.”
Roxane Lahidji, “Marbled Salts” - https://www.roxanelahidji.com/marbled-salts, accessed August 14, 2020.
Weiwei Zhang et al., “Unexpected Stable Stoichiometries of Sodium Chlorides,” Science 342, no. 6165 (2013) - doi:10.1126/science.1244989.
Faulders Studio, “Geotube Tower.”
Manor et al., Sigalit Landau.
Meinhold, “Dead Sea ‘Cristal’ Pavilion.”
Red Dot Design Award, “Salt Crystal Wall.”
Sibbel, “SEAt.”
Manor et al., Sigalit Landau.
Sibbel, “SEAt.”
Royal Institute of British Architects, “Presidents Medals: An Augmented Ecology of Wildlife and Industry,” Royal Institute of British Architects - http://www.presidentsmedals.com/Entry-23601, accessed June 8, 2020.
Faulders Studio, “Geotube Tower.”
Meinhold, “Dead Sea ‘Cristal’ Pavilion.”
Red Dot Design Award, “Salt Crystal Wall.”
This work was partly supported by the research project Building with Salt of the Fritz and Trude Fortmann Foundation for Building Culture and Materials, Germany between 08.2019 and 08.2020.
The authors gratefully thank the Laboratory of Building Structures, Division Building Materials and Construction Chemistry at the Technical University Munich for their support.
The authors want to state the following authorship items:
- Vesna Pungercar: conceptualization, methodology, writing - original draft;
- Florian Musso: review (all chapters).
All figures and tables: © Vesna Pungercar.
Vesna Pungercar is a Research Associate and PhD Candidate at the Chair of Building Construction and Material Science EBB, Technical University of Munich (TUM). She works and coordinates research projects on sustainable construction, building materials, and building technology, which have been published in scientific journals and international conferences. E-mail: vesna.pungercar@tum.de
Florian Musso is a full Professor in Building Construction and Material Science (EBB) at the Technical University of Munich, Munich 80333, Bayern, Germany TUM. He carries out research in the fields of construction materials and subsystems in industrial construction and runs an architectural practice LorenzMusso Architects in Sion/CH and Munich.
E-mail: musso@tum.de